Research topics
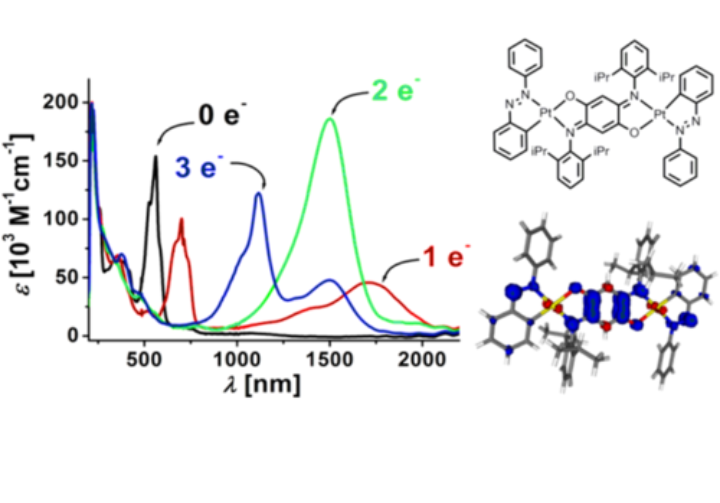
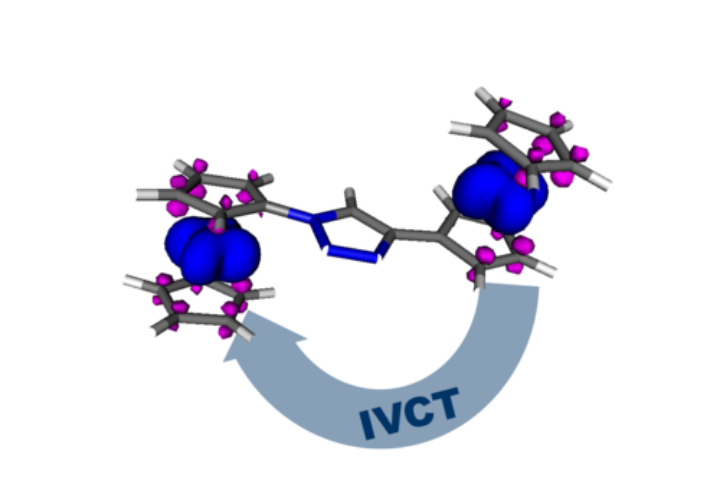
Complexes that display electrochromic behaviour are ideal starting materials for optically switchable devices. In this project, we aim to combine the redox and optical properties of non-innocent ligands to obtain metal complexes with strong, redox-switchable NIR absorption bands. A long-term goal is to transfer these processes to surfaces to develop smart optical windows.
Bidentate and tripodal ligands bearing triazole-based donor functionalities can be synthesised in high yields with excellent selectivity be means of the CuAAC "Click" reaction. The resulting metal complexes are competent catalysts for C-H oxygenation and amination reactions, N-arylations as well as for olefine polymerisation and oligomerisation.
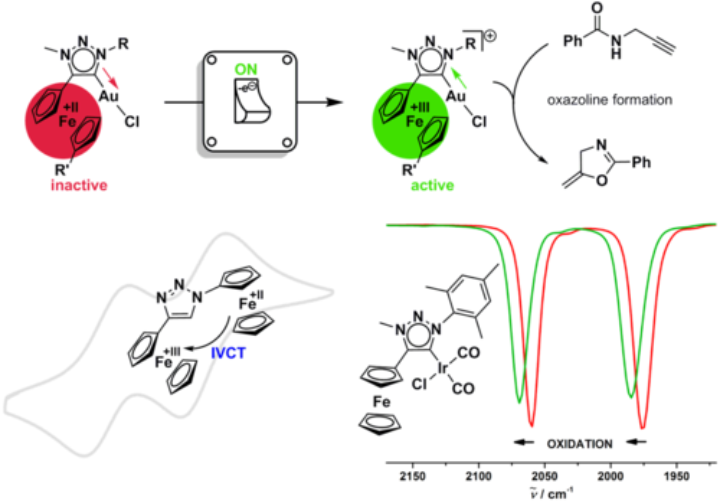
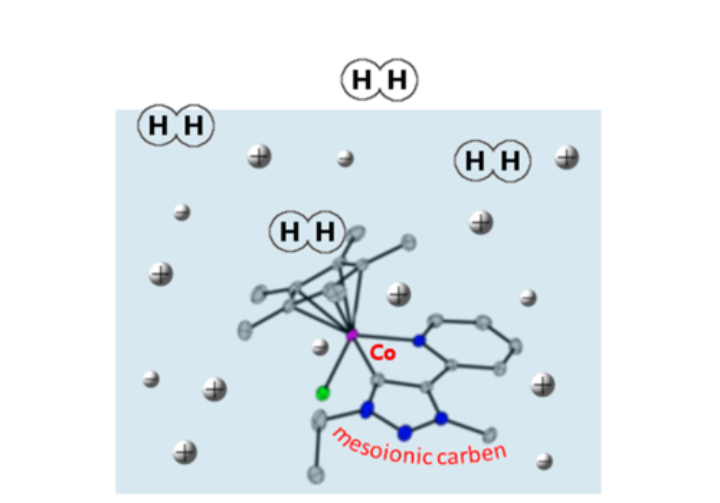
The use of meso-ionic carbenes, a novel class of carbene ligands, as powerful ligands in metal catalysed reactions has strongly increased in recent years. We were able to show that such metal complexes serve as highly efficient catalysts for a variety of reactions, e. g. C-C cross couplings, C-H oxygenation cycloaddition, transfer hydrogenations as well as α-arylation of amides. Our current focus is on the synthesis of multinuclear complexes to investigate possible catalytic cooperativity between the metal centres.
In contrast to most of our catalytically active systems, this project puts emphasis on making use of the ligand's electrons and protons for bond formation and activation reactions. Recently, we have accomplished (electro-)catalytical C-C bond formation, reduction of C=O functionalities and cyclisation reaction using these unconventional catalytic systems. The project aims at using electrons of the metal centre and the non-innocent ligand in a cooperative fashion to obtain active homogenous catalysts.
Nature has mastered the art of using non-innocent ligands in highly effective transition metal complexes that allow the activation and chemical transformation of small molecules. Inspired by these enzymatic processes, we try to use quinoid systems and meso-ionic carbenes in metal complexes as electron-reservoirs for both the activation and formation of H2 as well as for the activation of O2, CO2 and H2O. By incorporatong non-innocent ligands into the metal complexes, multi-electron and proton transfer reactions are facilitated and thereby the desired reactions made possible. Furthermore, we try to extend these activation reactions to catalytic transformations that allow for the production of useful chemicals.
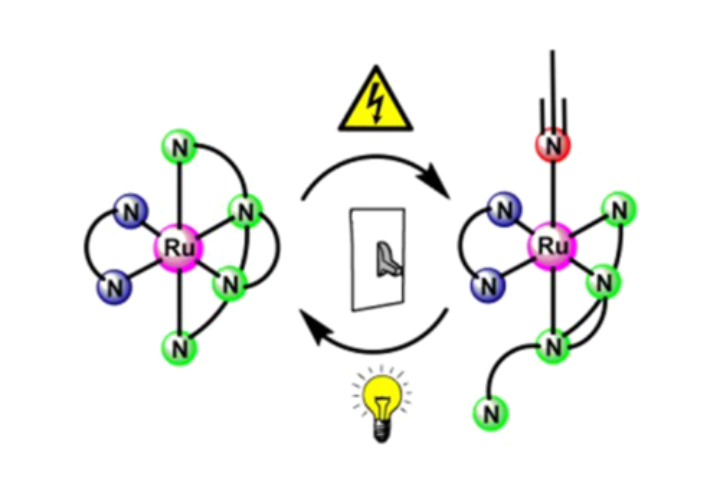
Triazolhaltige tripodale Liganden welche mithilfe der „Click“-Chemie hergestellt werden, bieten eine ideale Koordinationsumgebung für photochemische und elektrochemische Bindungsaktivierungsreaktionen an Metallzentren. In diesem Projekt versuchen wir die elektrochemische und photochemische Reaktivität durch die gezielte Variation der sterischen und elektronischen Eigenschaften der tripodalen Liganden einzustellen. Anspruchsvolle photochemische Messungen werden teilweise durch unsere Kollaborationspartner durchgeführt. Triazole-based tripodal ligands, synthesized via "Click" chemistry, offer an ideal coordination environment for photo- and electrochemical bond activation reactions at metal centres. We try to tune the electro- and photochemical reactivity via targeted variation of the sterixc and electronic properties of the tripodal ligands. Complex photochemical measurements are performed by our collaborators, mostly within the DFG-funded priority programme "Light Controlled Reactivity of Metal Complexes" (SPP2102)
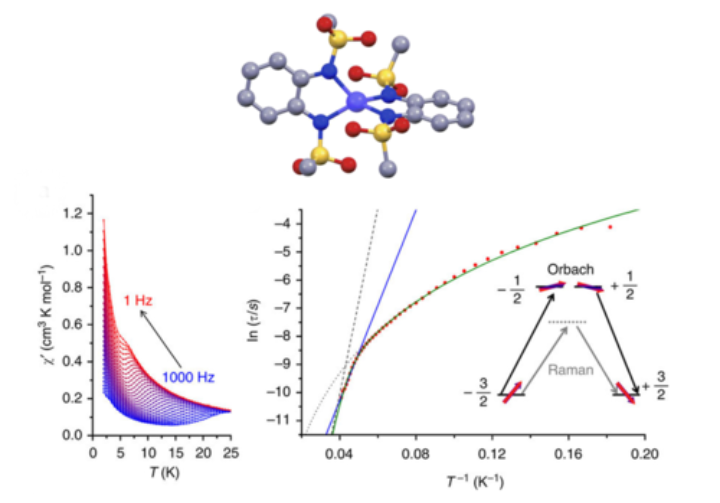
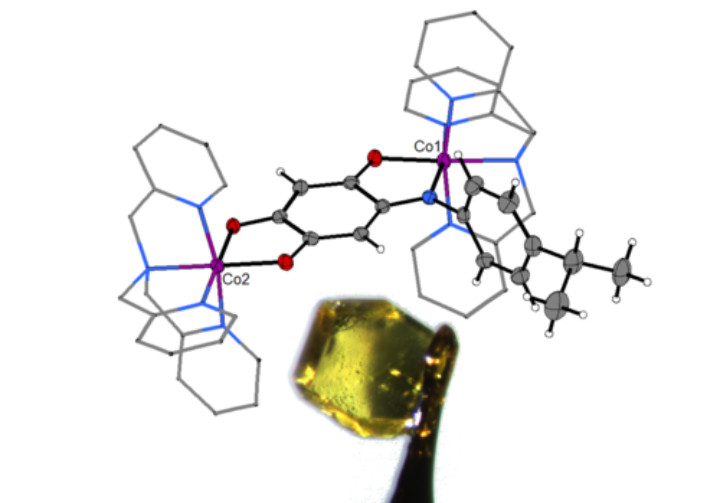
We believe that ligand systems such as quinones, triazoles and meso-ionic carbenes have great potential in devising novel magnetically switchable molecular materials. While earlier work resulted in mononuclear Co(II) and Fe(II) complexes displaying SCO behaviour with a large hysteresis loop close to room temperature, we recently extended our work on Co(II)-based Single Molecule Magnets to dinuclear systems in order to investigate relaxation processes. These projects entail a close collaboration with groups that are specialised in molecular magnetism and theoretical chemistry. These fascinating molecules might be used as molecular data storage devices in future applications.
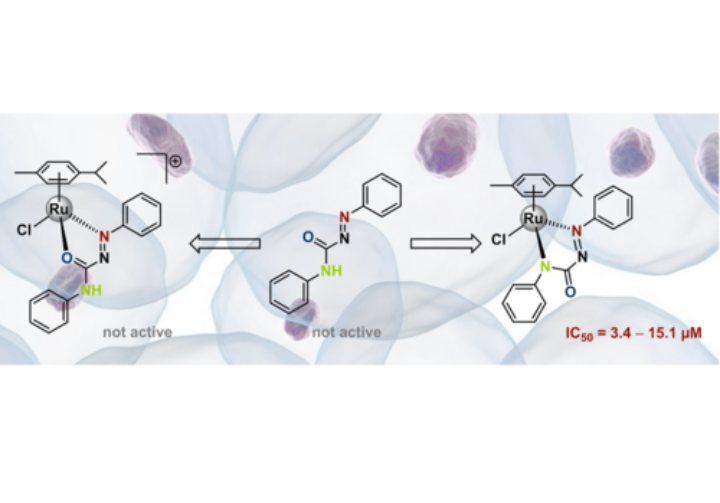
A new research topic in our group is the use of metal complexes bearing diimines, triazoles and meso-ionic carbenes for (potential) applications in anti-tuomr research or as antibacterial agents. This projects is worked on in conjunction with biochemists and medicinal chemists.
Methods
We apply organic methodology to synthesize tailor-made ligand systems. These are subsequently used to form metal complexes with a defined coordination sphere. Principles from organometallic and traditional coordination chemistry as well as photochemistry and microwave-assisted synthesis are employed in this regard. This holistic approach allows for the targeted synthesis of catalytically active or switchable complexes. Reactive species and catalytic intermediates can be accessed by working under inert conditions.
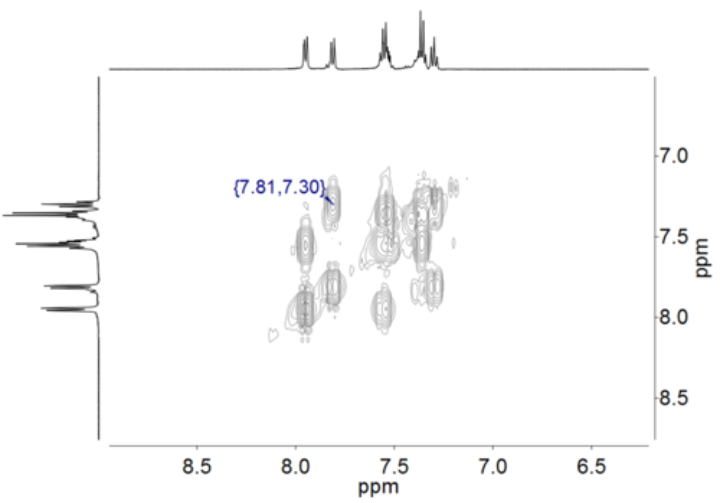
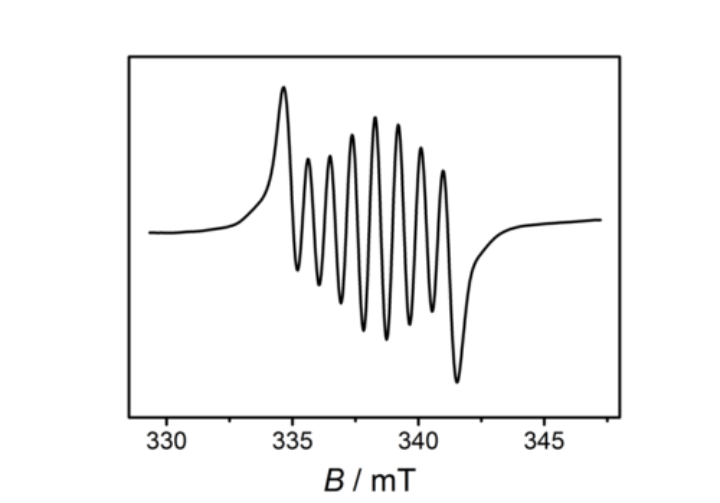
Initial characterisation of new compounds is often performed via one-dimensional 1H and 13C NMR spectroscopy. If the corresponding nuclei are present, this is complemented by e. g. 31P, 19F, or 15N spectra. Multi-dimensional NMR spectroscopy is used for the precise assignment of nuclei in more complex molecules. Additionally, these methods are sued to detect and characterise intermediates in catalyse, which can lead to important mechanistic insights. Also in the case of paramagnetic substances, temperature-dependent measurements are invaluable to gather information on spin state and possible spin state witching in solution.
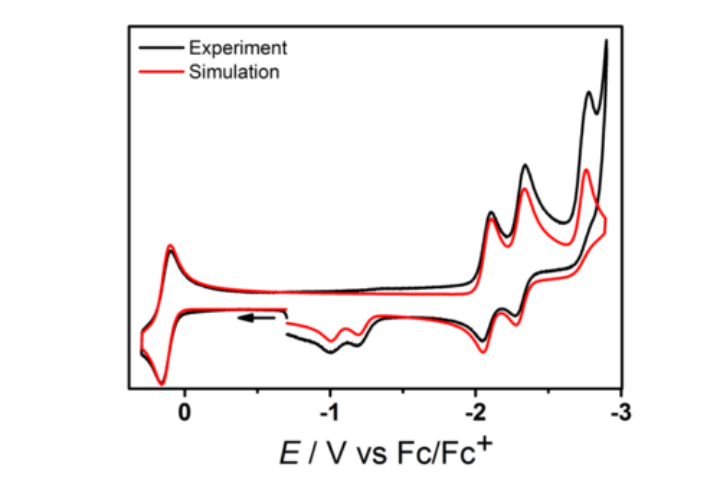
Redox-active organic compounds and their metal complexes are at the heart of our research. In order to elucidate electron transfer processes, we use a combination of CV and DPV. The same methods are also employed to investigate electrocatalytic processes such H2 activation and production, CO2 reduction and C-C bond formation. These methods are supplemented by bulk electrolysis. Our current focus is the use of electrochemical methods to investigate the reaction mechanism of electrocatalytic reactions.
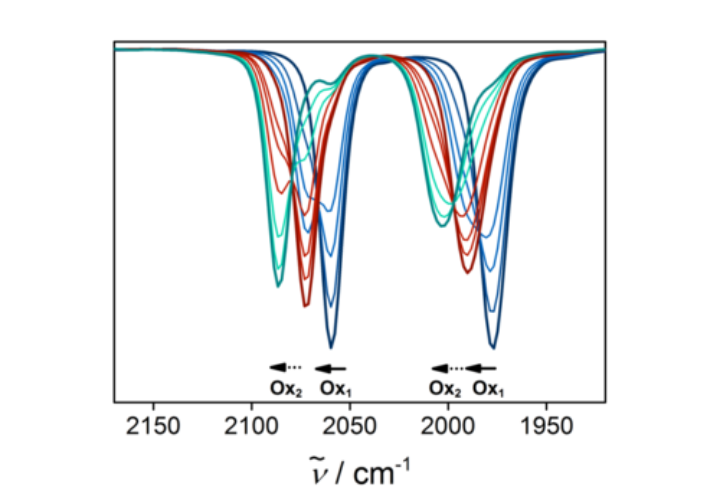
Besides NMR spectroscopy, IR spectroscopy is extremely powerful for gaining structural information on molecules. Often this method is used as a simple characterization tool. However, we also use IR spectroscopy in combination with electrochemistry (IR spectroelectrochemistry). Such an approach allows us to gather structural information on chemical systems in their various redox states. IR spectroelectrochemistry also provides useful information on the charge distribution in molecules. Additionally, IR spectroelectrochemistry helps us in determining the donor properties of new redox-active ligands and in probing mechanisms of electrocatalytic reactions.
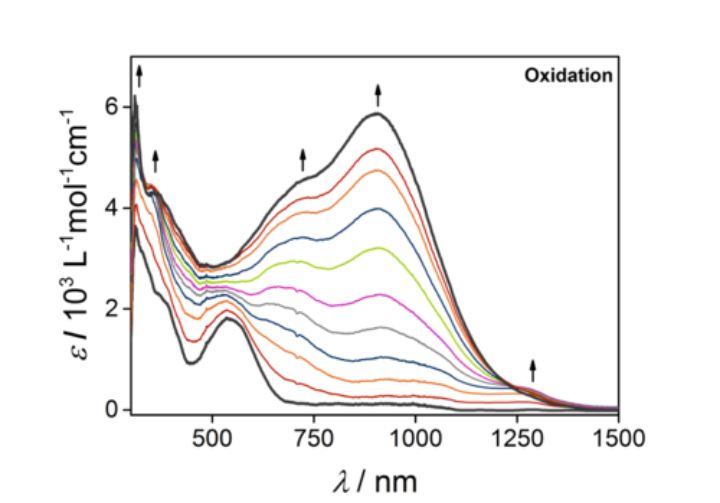
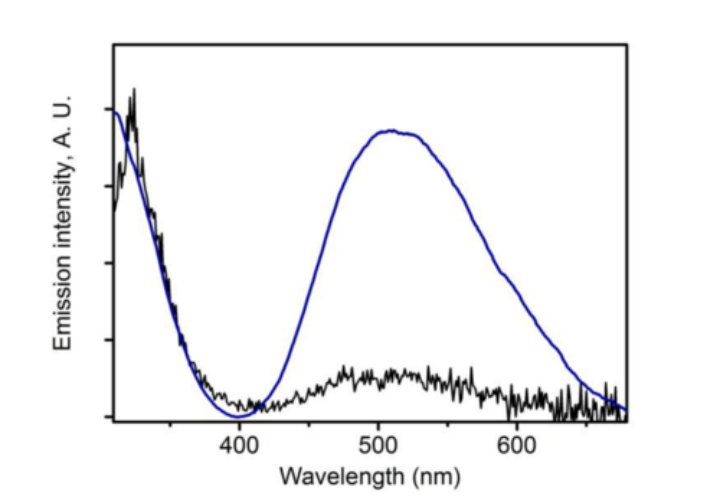
Many compounds synthesized in our labs are strongly colored. Hence, absorption spectroscopy in the visible region is extremely useful for the characterization of such compounds. As one focus of our research is the generation of visible and NIR electrochromic dyes, the combination of electrochemistry and UV-vis-NIR spectroscopy (spectroelectrochemistry) is widely used in our labs. This combination allows us to determine optical switching in the visible and the NIR region. Additionally, we use UV-vis-NIR spectroelectrochemistry to gain information on the electronic structures of the compounds in their various redox states, to investigate electron transfer processes, and to investigate electrocatalytic reaction mechanisms.
For an inorganic chemist, the epitome of structural characterization is single crystal X-ray diffraction. We often characterize all our metal complexes through this method. In cases, where information from spectroscopic methods are not sufficient for the prediction of the structure of a new compound, information from single crystal X-ray diffraction is extremely useful. Additionally, for paramagnetic compounds, this method delivers direct structural information on the samples, which is otherwise hard to obtain through other methods. In some cases, intermediates obtained from chemical reactions are investigated with single crystal X-ray diffraction to obtain valuable information about the reaction mechanism.
In modern chemistry, DFT calculations play a vital role in elucidating diverse chemical and physical properties of chemical compounds. In our group, DFT calculations are used to probe the electronic structures of metal complexes, and to complement and comprehend the spectroscopic data obtained from several spectroscopic techniques. Such a combined theoretical and experimental approach helps us in understanding our systems better, and also provides us with some predictive power over newly synthesized systems. While basic DFT is carried out in the group itself, we collaborate with expert theoreticians on advanced problems.
Light is an extremely powerful, benign and environmentally friendly energy source for carrying out various chemical reactions including catalysis. In our laboratories, we use light for both catalysis, as well as for bond activation reactions resulting in highly reactive and exotic chemical species. In many cases, it is not possible to carry out these light-induced reactions through normal thermal activations. A first focus of these investigations is to convert light into useful chemistry. However, the mid- and long-term goals of these projects are to understand the photochemical landscapes of such species, and to correlate these with the observed chemical reactivity. Many of the spectroscopic methods mentioned above are combined with photochemical reactions to probe mechanisms. Additionally, we collaborate with photochemists and photophysicists to have a closer look at the excited states of these molecules.